
Publications
“Modeling of Cu(II)-based protein spin labels using rotamer libraries“, Z. Hasanbasri, M. Tessmer, S. Stoll, and S. Saxena, Phys. Chem. Chem. Phys., 26 (2024) 6806. (link)
“Orientational selectivity in pulsed EPR does not have to be complicated”, Z. Hasanbasri and S. Saxena, Appl. Magn. Reson., 55 (2024) 61. (link)
“Integrating electron paramagnetic resonance spectroscopy and computational modeling to measure protein structure and dynamics“, X. Bogetti and S. Saxena, ChemPlusChem., 89 (2024) e202300506. (link)
“Differentiating between label and protein conformers in pulsed dipolar EPR spectroscopy with the dHis-Cu2+(NTA) motif“, C. A. Heubach, Z. Hasanbasri, D. Abdullin, A. Reuter, B. Korzekwa, S. Saxena, and O. Schiemann, Chem. Eur. J., 29 (2023) e202302541. (link)
“Double quantum coherence ESR at Q-band enhances the sensitivity of distance measurements at sub-micromolar concentrations”, A. Mandato, Z. Hasanbasri, and S. Saxena, J. Phys. Chem. Lett., 14 (2023) 8909. (link)
“Direct observation of negative cooperativity in a detoxification enzyme at the atomic level by EPR and simulation”, X. Bogetti, A. Bogetti, J. Casto, G. Rule, L. Chong, and S. Saxena, Prot. Sci., 32 (2023) e4770. (link)
“Efficient sampling of molecular orientations for Cu(II)-based DEER on protein labels”, Z. Hasanbasri, N. Moriglioni, and S. Saxena, Phys. Chem. Chem. Phys., 25 (2023) 13275. (link) (This paper was selected as a journal cover.)
“‘Store bought is fine’: Sensitivity considerations using shaped pulses for DEER measurements on Cu(II) labels”, J. Casto, X. Bogetti, H. Hunter, Z. Hasanbasri, and S. Saxena, J. Magn. Reson., 349 (2023) 107413. (link)
“A new 13C trityl-based spin label enables the use of DEER for distance measurements”, Z. Hasanbasri, M. Poncelet, H. Hunter, B. Driesschaert, and S. Saxena, J. Magn. Reson., 347 (2023) 107363. (link)
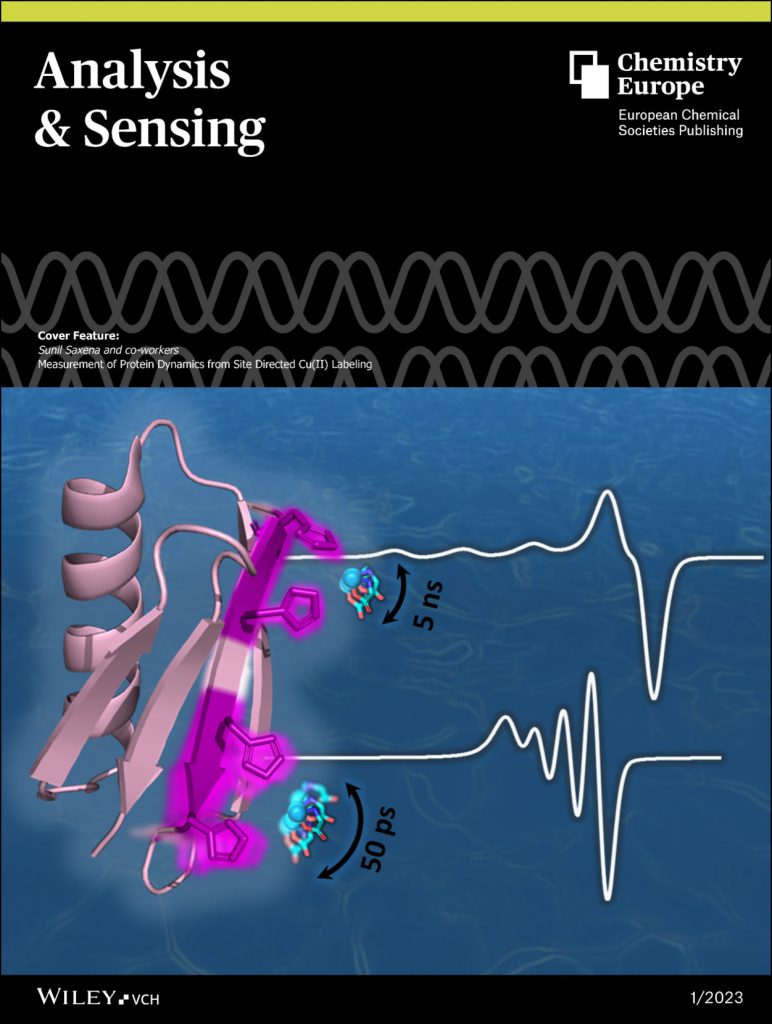
“Measurement of protein dynamics from site directed Cu(II) Labeling”, K. Singewald, H. Hunter, T. F. Cunningham, S. Ruthstein, and S. Saxena, Analysis & Sensing, 3 (2023) e202200053. (link) (This paper was selected as a journal cover.)
“The use of EPR spectroscopy to study transcription mechanisms”, L. Hofmann, A. Mandato, S. Saxena, and S. Ruthstein, Biophys. Rev., 14 (2022) 1141. (link)

“Synthesis of square planar Cu4 clusters”, M. K. Osei, S. Mirzaei, X. Bogetti, E. Castro, M. A. Rahman, S. Saxena, and R. Hernández-Sánchez, Angew. Chem. Intl. Ed. 61 (2022) e202209529. (link)
“Beyond structure: Deciphering site-specific dynamics in proteins from double histidine based EPR measurements”, K. Singewald, J. A. Wilkinson, Z. Hasanbasri, S. Saxena, Prot. Sci. 31 (2022) e4359. (link)
“An optimal acquisition scheme for Q-band EPR distance measurements using Cu2+-based protein labels”, X. Bogetti, Z. Hasanbasri, H. R. Hunter and S. Saxena, Phys. Chem. Chem. Phys. 24 (2022) 14727. (link) (This paper was selected as a journal back cover.)
“Allostery-driven changes in dynamics regulate the activation of bacterial CueR copper transcription factor”, I. Yakobov, A. Mandato, L. Hofmann, K. Singewald, Y. Shenberger, L. Gevorkayn-Airapetov, S. Saxena, and S. Ruthstein, Prot. Sci. 31 (2022) e4309. (link)
“Cu2+-based DNA labeling identifies the structural link between transcriptional activation and termination in a metalloregulator”, J. Casto, A. Mandato, L. Hofmann, I. Yakobov, S. Ghosh, S. Ruthstein, and S. Saxena, Chem. Sci. 13 (2022) 1693. (link)
“Structures of highly flexible intracellular domain of human α7-nicotinic acetlycholine receptor”, V Bondarenko, M. M. Wells, Q. Chen, T. S. Tillman, K. Singewald, M. J. Lawless, J. Caporoso, N. Brandon, S. Saxena, E. Lindahl, Y. Xu, and P. Tang, Nat. Comm. 13 (2022) 793. (link) (This paper was selected as an Editor’s Highlight.)
“Copper based site-directed spin labeling of proteins for use in pulsed and continuous wave EPR spectroscopy”, K. Singewald, J. A. Wilkinson, and S. Saxena, Bio-Prot. 11 (2021) e4258. (link)

“Nanoscale spin detection of CuCl2 molecules using double-electron resonance at room temperature”, K. Zhang, S. Ghosh, S. Saxena, and G. Dutt, Phys. Rev. B 104 (2021) 224412. (link)
“Benchmark test and guidelines for DEER/PELDOR experiments on nitroxide-labeled biomolecules”, O. Schiemann, C. A. Heubach, D. Abdullin, K. Ackermann, M. Azarkh, E. Bagryanskaya, M. Drescher, B. Endeward, J. H. Freed, L. Galazzo, D. Goldfarb, T. Hett, L. E. Hofer, L. F. Ibanez, E. J. Hustedt, S. Kucher, I. Kuprov, J. E. Lovett, A. Meyer, S. Ruthstein, S. Saxena, S. Stoll, C. Timmel, M. D. Valentin, H. S. Mchaourab, T. F. Prisner, B. E. Bode, E. Bordignon, M. Bennati, and G. Jeschke, J. Am. Chem. Soc. 143 (2021) 17875. (link)
“dHis-troying barriers: Deuteration provides a pathway to increase sensitivity and accessible distances for Cu2+ labels”, J. Casto, A. Mandato, and S. Saxena, J. Phys. Chem. Lett. 12 (2021) 4681. (link)
“Cleavage-resistant protein labeling with hydrophilic trityl enables distance measurements in-cells”, Z. Hasanbasri, K. Singewald, T. D. Gluth, B. Driesschaert, and S. Saxena, J. Phys. Chem. B. 125 (2021) 5265. (link)
“Going the dHis-tance: Site-directed Cu2+ labeling of proteins and nucleic acids”, A. Gamble Jarvi, X. Bogetti, K. Singewald, S. Ghosh, S. Saxena, Acc. Chem. Res. 54 (2021) 1481. (link) (This paper was selected as a supplementary journal cover.)
“Orientation and dynamics of Cu2+ based DNA label from force field parameterized MD elucidates the relationship between EPR distance constraints and DNA backbone distances”, S. Ghosh, J. Casto, X. Bogetti, C. Arora, J. Wang, S. Saxena, Phys. Chem. Chem. Phys. 22 (2020) 26707. (link) (This paper was selected as a journal cover and featured in the 2020 PCCP Hot Articles themed collection.)


“H2/CO2 Separations in Multicomponent Metal-Adeninate MOFs with Multiple Chemically Distinct Pore Environments”, Z. M. Schulte, Y. H. Kwon, Y. Han, C. Liu, L. Li, Y. Yang, A. Gamble Jarvi, S. Saxena, G. Veser, J. K. Johnson, and N. L. Rosi, Chem. Sci. 11 (2020) 12807. (link)
“Buffer effects on site directed Cu2+-labeling using the double histidine motif”, A. Gamble Jarvi, J. Casto, and S. Saxena, J. Magn. Reson. 320 (2020) 106848. (link)
“Double Histidine based EPR measurements at physiological temperatures permit site-specific elucidation of hidden dynamics in enzymes”, K. Singewald, X. Bogetti, K. Sinha, G. Rule, and S. Saxena, Angew. Chem. Intl. Ed. 59 (2020) 23040. (link)
“Development of Cu2+-based distance methods and force field parameters for the determination of PNA conformations and dynamics by EPR and MD simulations”, A. Gamble Jarvi, A. Sargun, X. Bogetti, J. Wang, C. Achim, and S. Saxena, J. Phys. Chem. B. 124 (2020) 7544. (link) (This paper was selected as a journal cover.)
“Molecular dynamics simulations based on newly developed force field parameters for Cu2+ spin labels provide insights into double Histidine based Double Electron Electron Resonance”, X. Bogetti, S. Ghosh, A. Gamble Jarvi, J. Wang, and S. Saxena, J. Phys. Chem. B. 124 (2020) 2788. (link) (This paper was selected as a journal cover.)


“Cu2+-based distance measurements by pulsed EPR provide distance constraints for DNA backbone conformations in solution”, S. Ghosh, M. Lawless, H. Brubaker, K. Singewald, M. Kurpiewski, L. Jen-Jacobson, and S. Saxena, Nucleic Acids Res. 48 (2020) e49. (link)
“Structural and dynamic origins of ESR lineshapes in spin-labeled proteins: the insights from spin dynamics simulations based on long MD trajectories”, S. A. Izmailov, S. O. Sevastyan, Z. Hasanbasri, I. S. Podkorytov, S. Saxena, and N. R. Skrynnikov, Sci. Rep. 10 (2020) 957. (link)
“19F paramagnetic relaxation-based NMR for quaternary structural restraints of ion channels”, V. Bondarenko, M. M. Wells, Q. Chen, K. C. Singewald, S. Saxena, Y. Xu, and P. Tang, ACS Chem. Biol. 14 (2019) 2160. (link)
“Designing open metal sites in metal-organic frameworks for paraffin/olefin separations”, M. H. Mohamed, Y. Yang, L. Li, S. Zhang, J. P. Ruffley, A. Gamble Jarvi, S. Saxena, G. Veser, K. J. Johnson, and N. Rosi, J. Am. Chem. Soc. 141 (2019) 13003. (link)
“An undergraduate experiment to explore Cu(II) coordination environment in multi-histidine compounds through electron spin resonance spectroscopy”, E. P. Wagner, K. C. Gronborg, S. Ghosh, and S. Saxena, J. Chem. Educ. 96 (2019) 1752. (link)
“Understanding and controlling the metal-directed assembly of terpyridine-functionalized coiled-coil peptides”, K. A. Scheib, N. A. Tavenor, M. J. Lawless, S. Saxena, and W. S. Horne, Chem. Comm. 55 (2019) 7752. (link)
“Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions”, J. Huang, Y. Dai, K. Singewald, C. C. Liu, S. Saxena, and H. Zhang, Chem. Eng. J. 370 (2019) 906. (link)
“Efficient localization of a native metal ion within a protein by Cu2+-based EPR distance measurements”, A. Gamble Jarvi, T. F. Cunningham, and S. Saxena, Phys. Chem. Chem. Phys. 21 (2019) 10238. (link) (This paper was selected as a featured inside cover and featured in the 2019 PCCP Hot Articles themed collection.)
“EPR spectroscopy detects various active state conformations of the transcriptional regulator CueR”, H. Sameach, S. Ghosh, L. Gevorkyan-Airapetov, S. Saxena, and S. Ruthstein, Angew. Chem. Intl. Ed. 58 (2019) 3053. (link) (This paper was selected as a journal cover.)


“Increasing nitroxide lifetime in cells to enable in-cell protein structure and dynamics measurements by electron spin resonance spectroscopy”, K. Singewald, M. J. Lawless, and S. Saxena, J. Magn. Reson. 299 (2019) 21. (link) (This paper was selected as a journal cover.)
“On the use of Q-band DEER to resolve the relative orientations of two double histidine bound Cu2+ ions in a protein”, A. Gamble Jarvi, K. Ranguelova, S. Ghosh, R. Weber, and S. Saxena, J. Phys. Chem. B. 122 (2018) 10669. (link) (This paper was selected as a supplementary journal cover.)


“Evolution of surface Copper(II) environments in Cu2-xSe nanoparticles”, D. C. Kaseman, A. Gamble Jarvi, X. Y. Gan, S. Saxena, J. E. Millstone, Chem. Mater. 30 (2018) 7313. (link)
“Cu(II) EPR reveals two distinct binding sites and oligomerization of innate immune protein calgranulin C”, S. Ghosh, V. Garcia, K. Singewald, S. Damo, S. Saxena, Appl. Magn. Reson. 49 (2018) 1299. (link)
“Rotamer modelling of Cu(II) spin labels based on the double-histidine motif”, S. Ghosh, S. Saxena, G. Jeschke, Appl. Magn. Reson. 49 (2018) 1281. (link)
“Programmable topology in new families of heterobimetallic metal-organic frameworks”, P. F. Muldoon, C. Liu, C. C. Miller, S. B. Koby, A. Gamble Jarvi, T. Y. Luo, S. Saxena, M. O’Keeffe, N. L. Rosi, J. Am. Chem. Soc. 140 (2018) 6194. (link)
“ESR resolves the C terminus structure of the ligand-free human glutathione S-transferase A1-1”, M. J. Lawless, J. R. Petersson, G. S. Rule, F. Lanni, S. Saxena, Biophys J. 114 (2018) 592. (link)
“The Cu2+-nitrilotriacetetic acid complex improves loading of α-helical double histidine sites for precise distance measurements by pulsed ESR”, S. Ghosh, M. J. Lawless, G. S. Rule, S. Saxena, J. Magn. Reson. 286 (2018) 163. (link)
“On the use of the Cu2+-iminodiacetic acid complex for double histidine based distance measurements by pulsed ESR”, M. J. Lawless, S. Ghosh, T. F. Cunningham, A. Shimshi, S. Saxena, Phys. Chem. Chem. Phys. 19 (2017) 20959. (link)
“Analysis of nitroxide-based distance measurements in cell extracts and in cells by pulsed ESR spectroscopy”, M. J. Lawless, A. Shimshi, T. F. Cunningham, M. N. Kinde, P. Tang, S. Saxena, ChemPhysChem 18 (2017) 1653. (link)
“Nucleotide-independent Copper(II)-based distance measurements in DNA by pulsed ESR spectroscopy”, M. J. Lawless, J. L. Sarver, S. Saxena, Angew. Chem. Intl. Ed. 56 (2017) 2115. (link)
“Rotameric preferences of a protein spin label at edge-strand β-sheet sites”, T. F. Cunningham, S. Pornsuwan, W. S. Horne, S. Saxena, Prot. Sci. 25 (2016) 1049. (link)
“Cu(II)-Zn(II) cross-modulation in Amyloid-β peptide binding: An X-ray absorption spectroscopy study”, E. D. Santis, V. Minicozzi, O. Proux, G. Rossi, K. I. Silva, M. J. Lawless, F. Stellato, S. Saxena, S. Morante, J. Phys. Chem. B 119 (2015) 15813. (link)
“Cu2+ as an ESR probe of protein structure and function”, Z. Yang, M. Ji, T. F. Cunningham, S. Saxena, Methods. Enzymol. 563 (2015) 459. (link)
“A simple double quantum coherence ESR sequence that minimizes nuclear modulations in Cu2+-ion based distance measurements”, S. Ruthstein, M. Ji, B. K. Shin, S. Saxena, J. Magn. Reson. 257 (2015) 45. (link)
“Conformational changes underlying desensitization of the pentameric ligand-gated ion channel ELIC”, M. N. Kinde, Q. Chen, M. J. Lawless, E. Seyoum, D. D. Mowrey, J. Xu, V. Bandarenko, T. S. Tillman, S. Saxena, Y. Xu, P. Tang, Structure. 23 (2015) 995. (link)
“The double Histidine Cu2+-binding motif: A highly rigid, site-specific spin probe for ESR distance measurements”, T. F. Cunningham, M. R. Putterman, A. Desai, W. S. Horne, S. Saxena, Angew. Chem. Intl. Ed. 54 (2015) 6330. (link)
“Cysteine specific Cu2+-chelating tags used as paramagnetic probes in double electron electron resonance”, T. F. Cunningham, M. D. Shannon, M. R. Putterman, R. J. Arachchige, I. Sengupta, M. Gao, C. P. Jaroniec, S. Saxena, J. Phys. Chem. B 119 (2015) 2839. (link)
“Protein structural studies by paramagnetic solid-state NMR spectroscopy aided by a compact cyclen-type Cu(II) binding tag”, I. Sengupta, M. Gao, R. J. Arachchige, P. S. Nadaud, T. F. Cunningham, S. Saxena, C. D. Schweiters, C. P. Jaroniec, J. Biomol NMR 61 (2015) 1. (link)
“Origins of structural flexibility in protein-based supramolecular polymers revealed by DEER spectroscopy”, N. A. Tavenor, K. I. Silva, S. Saxena, S. Horne, J. Phys. Chem. B 118 (2014) 9881. (link)
“Insights into copper coordination in the EcoRI-DNA complex by ESR spectroscopy”, M. Ji, L. Tan, L. Jen-Jacobson, S. Saxena, Mol. Phys. 112 (2014) 3173. (link)
“ESEEM analysis of multi-histidine Cu(II)-coordination in model complexes, peptides and Amyloid-β”, K. I. Silva, B. C. Michael, S. J. Geib, S. Saxena, J. Phys. Chem. B 118 (2014) 8935. (link)
“Designing inhibitors of cytochrome c/cardiolipin peroxidase complexes: Mitochondria-targeted imidazole-substituted fatty acids”, J. Jiang, A. Bakan, A. Kapralov, Z. Huang, A. A. Amascato, V. K. Garapati, K. I. Silva, S. Saxena, H. Bayir, J. Atkinson, I. Bahar, V. E. Kagan, Free Radic. Biol. Med. 71 (2014) 221. (link)
“Paramagnetic metal ions in pulsed ESR distance distribution measurements”, M. Ji, S. Ruthstein, S. Saxena, Acc. Chem. Res. 47 (2014) 688. (link)
“Chemical and electrochemical manipulation of mechanical properties of stimuli-responsive copper-cross-linked hydrogels”, R. D. Harris, J. T. Auletta, S. A. M. Motlagh, M. J. Lawless, N. M. Perri, S. Saxena, L. M. Weiland, D. H. Waldeck, W. W. Clark, T. M. Meyer, ACS Macro Lett. 2 (2013) 1095. (link)
“Zn(II) ions substantially perturb Cu(II) ion coordination in Amyloid-β at physiological pH”, K. I. Silva, S. Saxena, J. Phys. Chem. B 117 (2013) 9386. (link)
“Sensitive Cu(II)-Cu(II) distance measurements in a protein-DNA complex by double-quantum coherence ESR”, S. Ruthstein, M. Ji, P. Mehta, L. Jen-Jacobson, S. Saxena, J. Phys. Chem. B 117 (2013) 6227. (link)
“Measuring Cu(II)-nitroxide distances using double electron-electron resonance and saturation recovery”, J. Sarver, K. I. Silva, and S. Saxena, Appl. Magn. Reson. 44 (2013) 583. (link)
“High-resolution structure of a protein spin-label in a solvent-exposed β-sheet and comparison with DEER spectroscopy”, T. F. Cunningham, M. S. McGoff, I. Sengupta, C. P. Jaroniec, W. Seth Horne and S. Saxena, Biochemistry 51 (2012) 6350. (link)
“Tunable, mixed-resolution modeling using library-based Monte Carlo and graphics processing units”, A. B. Manomov, S. Lettieri, Y. Ding, J. L. Sarver, R. Palli, T. F. Cunningham, S. Saxena, D. M. Zuckerman, J. Chem. Theory Comput. 8 (2012) 2921. (link)
“ESR spectroscopy identifies inhibitory Cu(II) sites in a DNA modifying enzyme to reveal determinants of catalytic specificity”, Z. Yang, M. Kurpiewski, M. Ji, J. E. Townsend, P. Mehta, L. Jen-Jacobson, and S. Saxena, Proc. Natl. Acad. Sci. 109 (2012) 6366. (link)
“Simulating the dynamics and orientations of spin-labeled side chains in a protein-DNA complex”, J. L. Sarver, J. E. Townsend, G. Rajapakse, L. Jen-Jacobson, and S. Saxena, J. Phys. Chem. B 116 (2012) 4024. (link)
“Insight into potential Cu(II)-binding motifs in the four pseudorepeats of tau protein”, B. K. Shin, S. Saxena, J. Phys. Chem. B 115 (2011) 15067. (link)
“Substantial contribution of the two imidazole rings of the His13-His14 dyad to Cu(II) binding in Amyloid-β1-16 at physiological pH and its significance”, B. K. Shin, S. Saxena, J. Phys. Chem. A 115 (2011) 9590. (link)
“Practical aspects of copper ion-based DEER distance measurements”, Z. Yang, M. Ji, S. Saxena, Appl. Magn. Reson. 39 (2010) 487. (link)
“Pulsed ESR resolves the coordination site of Cu(II) ions in α1-GlyR”, S. Ruthstein, K. M. Stone, T. F. Cunningham, M. Ji, M. Cascio, S. Saxena, Biophys. J. 99 (2010) 2497. (link)
“An approach to the measurement of nanometer range distances based on Cu(II) ions and ESR”, Z. Yang, D. Kise, S. Saxena, J. Phys. Chem. B 114 (2010) 6165. (link)
“The second Cu(II)-binding site in a proton rich environment interferes with the aggregation of Amyloid-β1-40 into amyloid fibrils”, S. Jun, J. R. Gillespie, B. Y. Shin, S. Saxena, Biochemistry 48 (2009) 10724. (link)
“Electron spin resonance analysis of the mechanism of pericyclic reactions of bicyclobutanes”, M. A. A. Walczak, B. K. Shin, P. Wipf, S. Saxena, Org. Biomol. Chem. 7 (2009) 2363. (link)
“Electron spin resonance shows common structural features for different classes of EcoRI-DNA complexes”, K. Stone, J. E. Townsend, J. Sarver, P. J. Sapienza, S. Saxena, L. Jen-Jacobson, Angew. Chem. Intl. Ed. 47 (2008) 10192. (link)
“Distance distributions of end-labeled curved bispeptide oligomers by electron spin resonance”, G. H. Bird, S. Pornsuwan, S. Saxena, C. E. Schafmeister, ACS Nano 2 (2008) 1857. (link)
“Direct evidence that all three histidine residues coordinate to Cu(II) in amyloid-β1-16”, B. K. Shin, S. Saxena, Biochemistry 47 (2008) 9117. (link)
“Analysis of the dynamical flexibility of bis-peptide nanostructures”, S. Pornsuwan, C. E. Schafmeister, S. Saxena, J. Phys. Chem. C 112 (2008) 1377. (link)
“On Cu(II)-Cu(II) distance measurement by pulsed electron-electron double resonance”, Z. Yang, J. S. Becker, S. Saxena, J. Magn. Reson. 188 (2007) 337. (link)
“The aggregated state of amyloid-β peptide in vitro depends on Cu(II) concentration”, S. Jun, S. Saxena, Angew. Chem. Intl. Ed. 46 (2007) 3959. (link)
“Lengths and flexibility of bis-peptide nanostructures using electron spin resonance”, S. Pornsuwan, G. Bird, C. E. Schafmeister, S. Saxena, J. Am. Chem. Soc. 128 (2006) 3876. (link)
“Unfolding of alanine-based peptides using electron spin resonance distance measurements”, S. Jun, J. Becker, M. Yonkunas, R. Coalson, S. Saxena, Biochemistry 45 (2006) 11666. (link)
“Double Quantum Coherence electron spin resonance on coupled Cu(II)-Cu(II) spins”, J. Becker, S. Saxena, Chem. Phys. Lett. 414 (2005) 248. (link)
“Binding of Cu(II) to thyrotropin-releasing hormone (TRH) and its analogs”, R. Meng, J. Becker, F. T. Lin, S. Saxena, S. G. Weber, Inorg. Chim. Acta. 358 (2005) 2933. (link)
“Suppression of electron spin echo envelope modulation peaks in double quantum coherence electron spin resonance”, M. Bonora, J. Becker, S. Saxena, J. Magn. Reson. 170 (2004) 278. (link)
“Nitroxide spin-relaxation over the entire motional range”, M. Bonora, S. Pornsuwan, S. Saxena, J. Phys. Chem. B 108 (2004) 4196. (link)
“Amplification of xenon NMR and MRI by remote detection”, A. J. Moule, M. M. Spence, S. I. Han, J. A. Seeley, K. L. Pierce, S. Saxena, A. Pines, Proc. Natl. Acad. Sci. 100 (2003) 9122. (link)
“Laser polarized 129Xe NMR and MRI at ultra-low magnetic fields”, A. Wong-Foy, S. Saxena, A. J. Moule, H. M. L. Bitter, J. A. Seeley, R. McDermott, J. Clarke, A. Pines, J. Magn. Reson. 157 (2002) 235. (link)
“Resolution of Xenon-129 chemical shifts at ultra low magnetic field”, S. Saxena, A. Wong-Foy, A. J. Moule, J. A. Seeley, R. McDermott, J. Clarke, A. Pines, J. Am. Chem. Soc. 123 (2001) 8133. (link)
“Solid state 2H-NMR studies of segmental dynamics in polymer blends”, S. Saxena, D. Cizmeyciyan, J. A. Kornfield, Solid State Nuclear Magnetic Resonance 12 (1998) 165. (link)
“Two-dimensional electron spin resonance and slow motions”, S. Saxena, J. H. Freed, J. Phys. Chem. A 101 (1997) 7998. (link)
“Theory of double quantum two dimensional electron spin resonance with application to distance measurements”, S. Saxena, J. H. Freed, J. Chem. Phys. 107 (1997) 1317. (link)
“Absorption lineshapes in two dimensional electron spin resonance and the effects of slow motions in complex fluids”, S. Saxena, J. H. Freed, J. Magn. Reson. 124 (1997) 439. (link)
“A theoretical approach to the analysis of arbitrary pulses in magnetic resonance”, K. M. Salikhov, D. J. Schneider, S. Saxena, J. H. Freed, Chem. Phys. Lett. 262 (1996) 17. (link)
“Double quantum two dimensional Fourier transform electron spin resonance: Distance measurements”, S. Saxena, J. H. Freed, Chem. Phys. Lett. 251 (1996) 102. (link)
“Non linear least-squares analysis of slow motional EPR spectra in one and two dimensions using a modified Levenberg-Marquardt Algorithm”, D. E. Budil, S. Lee, S. Saxena, J. H. Freed, J. Magn. Reson. A 120 (1996) 155. (link)
“Studies on lipid membranes by two dimensional Fourier transform ESR: Enhancement of resolution to ordering and dynamics”, R. H. Crepeau, S. Saxena, S. Lee, B. Patyal, and J. H. Freed, Biophys. J. 66 (1994) 1489. (link)
“Two-dimensional Fourier transform electron spin resonance in complex fluids”, S. Lee, B. R. Patyal, S. Saxena, R. H. Crepeau, J. H. Freed, Chem. Phys. Lett. 221 (1994) 397. (link)
Patents
“Remote NMR/MRI detection of laser polarized gases”, A. Pines, S. Saxena, A. Moule, M. Spence, J. A. Seeley, K. L. Pierce, S. I. Han, J. Granwehr, US Patent No. 7,061,237 (2006). (link)